The development of these principles arose from research into visualization strategies that might help reduce misconceptions in science students. These misconceptions, or incomplete conceptions, relate to molecular behaviours and properties of molecular environments. Visualizations created with 3D animation software have the capacity to imbue macromolecules with properties that may be close to or far from biophysical accuracy. A consideration of certain molecular behaviours when designing visuals may allow for a more robust understanding of the spaces in which these events take place.
Each principle is accompanied by a description and a pair of short animations. The illustrations depict both an example that does not adhere to the principle and one that does adhere to the principle. Above all, the primary objective of these illustrations is to demonstrate the principle with a high degree of clarity. Therefore, some of the other principles have been ignored or minimized to focus attention on the concept under consideration. While the examples shown are drawn from real biological processes and structures, many details of the processes have been omitted or simplified. Finally, we have chosen a consistent rendering style that balances a clean, simple presentation with plausible molecular representations.
Presenting molecular concepts to assist in the development of visualizations
Contributors
1

Molecules move through random collisions
Molecules move around through collisions resulting in random Brownian motion. In this example, in Treatment A, an Arp2/3 complex moves smoothly and linearly before binding to an actin filament, whereas in Treatment B, the motion is complex and chaotic.
To use this concept, consider depicting random walks.
Treatment A does not adhere to this principle, whereas Treatment B does. Expand the link below to watch the animations.
Principle 1 Animations
Treatment A
Treatment B
2
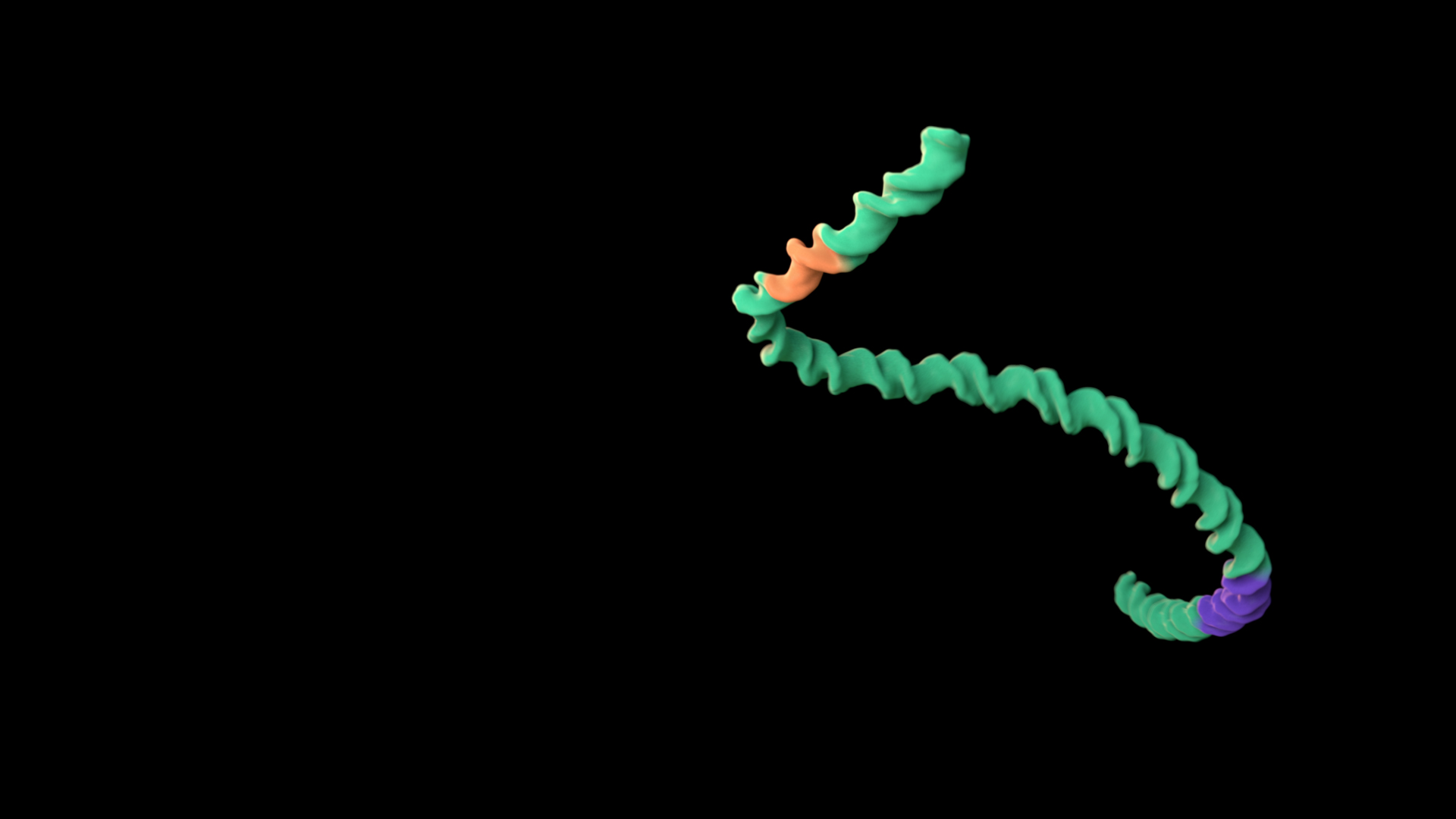
Long molecules experience similar forces along their length
The same forces are present along the full length of a long molecule. Putting a head to a molecule invokes agency. In this example, in Treatment A, a linear segment of double-stranded DNA appears to swim forward, whereas in Treatment B, it twists and turns without any “leading” end.
To use this concept, consider giving long molecules non-directional motion.
Treatment A does not adhere to this principle, whereas Treatment B does. Expand the link below to watch the animations.
Principle 2 Animations
Treatment A
Treatment B
3

Molecules are in constant motion
Newton’s first law states that objects remain in motion without external forces. While molecules are subjected to constant forces from all sides, the result is they are in constant motion and do not start and stop spontaneously. In this example, in Treatment A, CRAF, MEK, and ERK, components in a signalling cascade are not moving unless they are involved in binding, whereas in Treatment B, they continue moving, even when not immediately involved in the process.
To use this concept, consider keeping molecules moving.
Treatment A does not adhere to this principle, whereas Treatment B does. Expand the link below to watch the animations.
Principle 3 Animations
Treatment A
Treatment B
4

Intermolecular attractions are local forces
At this scale, showing negative pressure or distant molecules flooding toward a target invokes agency. The same applies to the relative motion between two binding partners. In this example, in Treatment A, sodium ions flow towards and traverse a voltage-gated ion channel as though attracted by a magnetic force, whereas in Treatment B, they move through the channel through local collisions and interactions with the transmembrane channel.
To use this concept, consider ensuring that distant molecules are unaffected by pulling forces.
Treatment A does not adhere to this principle, whereas Treatment B does. Expand the link below to watch the animations.
Principle 4 Animations
Treatment A
Treatment B
5

Unproductive collisions occur more often than productive ones
Not every encounter between complementary molecules results in binding; in fact statistically there are likely to be many more unproductive collisions than productive ones. In this example, in Treatment A, Sos and Ras come together and bind on first encounter, whereas in Treatment B, they collide a number of times before their orientations result in tight binding.
To use this concept, consider including non-binding collisions.
Treatment A does not adhere to this principle, whereas Treatment B does. Expand the link below to watch the animations.
Principle 5 Animations
Treatment A
Treatment B
6

Many instances of molecules and events exist
There are typically many instances of molecules and events present in a given environment; repetition can also reinforce the process being depicted. In this example, in Treatment A, only one copy each of siderocalin and enterobactin are shown, which bind together, whereas in Treatment B, there are several copies of each, and multiple binding events.
To use this concept, consider presenting multiple copies.
Treatment A does not adhere to this principle, whereas Treatment B does. Expand the link below to watch the animations.
Principle 6 Animations
Treatment A
Treatment B
7
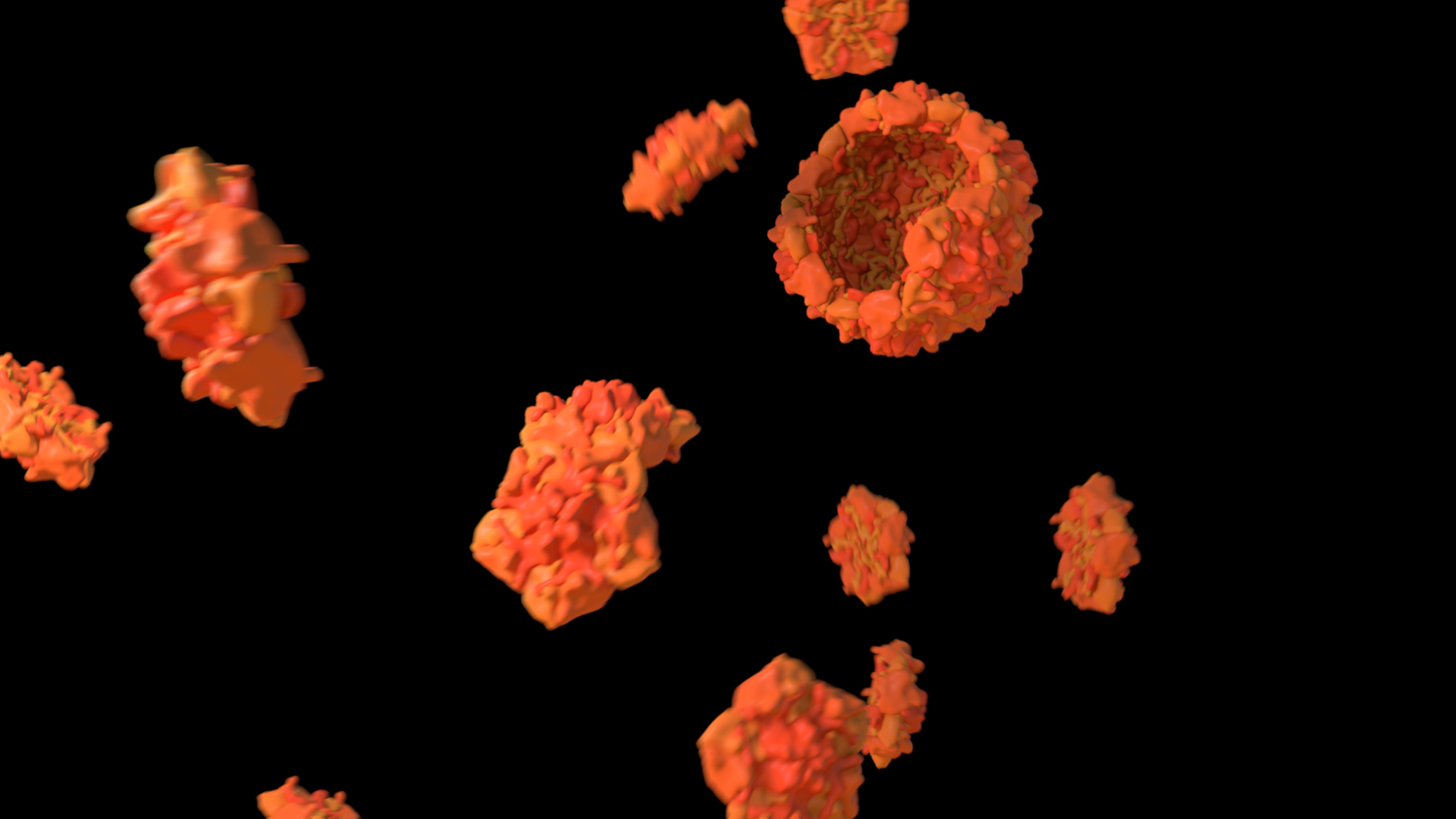
Not all instances of a molecule change state in a process
Not every molecule is used in a process or changes its state. More monomers are present than will be incorporated into a polymer, and typically more substrates are present than will be converted into a product. Likewise, not all molecules will cross a barrier or will bind to a chelator. In this example, in Treatment A, all subunits form a single viral capsid, whereas in Treatment B, once the capsid is complete, there are still subunits that remain.
To use this concept, consider leaving some molecules behind.
Treatment A does not adhere to this principle, whereas Treatment B does. Expand the link below to watch the animations.
Principle 7 Animations
Treatment A
Treatment B
8

Light and molecular water do not produce macroscopic phenomena
Water is composed of molecules and light does not interact with it at that scale to produce macroscopic phenomena, like caustics, refraction, distortion, or crepuscular/god rays. These “underwater” effects are not relevant at the molecular scale. In this example, in Treatment A, there are several post-processing effects that indicate an aqueous environment, whereas in Treatment B, molecular water is initially shown, and then faded out.
To use this concept, consider water as molecules.
Treatment A does not adhere to this principle, whereas Treatment B does. Expand the link below to watch the animations.
Principle 8 Animations
Treatment A
Treatment B
9

Molecular landscapes are crowded and diverse
Cellular environments are busy and crowded, with very little empty space, particularly if molecular water is included. Even without the depiction of molecular water, macromolecules take up a sizeable percentage of the volume. In this example, in Treatment A, a PTPS molecule moves in an intracellular environment consisting of actin filaments and a nucleus, whereas in Treatment B, a diverse suite of proteins, RNA, metabolites and more fill the surrounding cytoplasmic space.
To use this concept, consider populating molecular environments with macromolecules.
Treatment A does not adhere to this principle, whereas Treatment B does. Expand the link below to watch the animations.
Principle 9 Animations
Treatment A
Treatment B
10

Molecules are physical entities with definable boundaries
Molecules are physical entities with definable boundaries. Intersecting surface meshes provide conflicting or obscured information about interaction and binding sites. In this example, in Treatment A, a PCNA DNA clamp slides along a DNA strand with coarse meshing that overlaps with itself and with the DNA, whereas in Treatment B, the meshes are tightly defined and show space between the clamp and DNA, indicating intermolecular forces that keep the molecules from occupying the same space.
To use this concept, consider respecting atomic boundaries with surface meshes.
Treatment A does not adhere to this principle, whereas Treatment B does. Expand the link below to watch the animations.
Principle 10 Animations
Treatment A
Treatment B
11
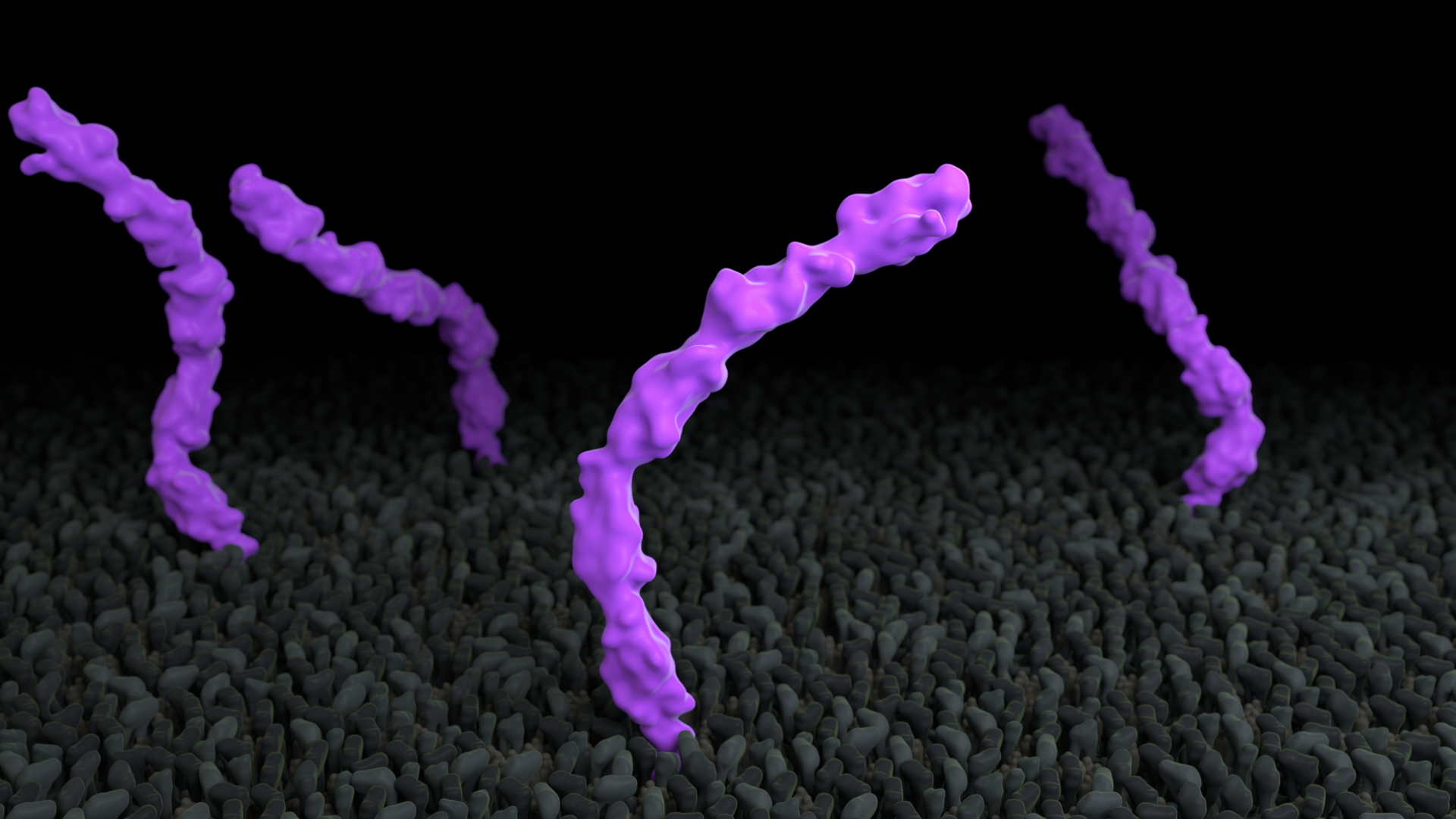
Proteins exhibit a range of flexibility
Proteins have internal freedom of motion that allows for specific functionality. Some parts of a protein are more flexible than others and some proteins are more rigid than others. In this example, in Treatment A, the membrane protein cadherin moves in the membrane, but is internally rigid, whereas in Treatment B, the five extracellular domains are linked by short flexible regions.
To use this concept, consider showing protein flexibility.
Treatment A does not adhere to this principle, whereas Treatment B does. Expand the link below to watch the animations.
Principle 11 Animations
Treatment A
Treatment B
12

Many binding reactions are reversible before reactions occur
Molecules do not bind permanently and many reactions are reversible at the individual molecule level. In this example, in Treatment A, the mTORC1 complex phosphorylates its substrate S6K1 the first time that it binds in every case, whereas in Treatment B, the substrate dissociates and binds again before being phosphorylated.
To use this concept, consider including some unproductive binding and dissociation.
Treatment A does not adhere to this principle, whereas Treatment B does. Expand the link below to watch the animations.
Principle 12 Animations
Treatment A
Treatment B
Funding





